Ceramic Handbook
Section outline
-
Clay
Clay is a flexible and tough material that can be formed using many different techniques. Moist and soft clay becomes hard and brittle as it dries, and when fired it hardens and condenses into a very durable material, i.e. ceramics. Clay materials are the oldest construction materials developed by humans from natural raw materials and, as such, raw clay has been used long before firing it for durability was invented.

Figure 1. Clay construction, Burkina Faso
 Figure 2. Different clay materials
Figure 2. Different clay materialsClay is a very fine, naturally occurring type of soil created by weathering of different types of rock. Clay can be found practically all over the world, in different places and in different colors. Different types of clay include e.g. kaolin, ball clays, red clays and other natural clays. Theoretically, geologists define clay as a type of mineral soil in which at least 30% of the particles are less than 0.002 mm.
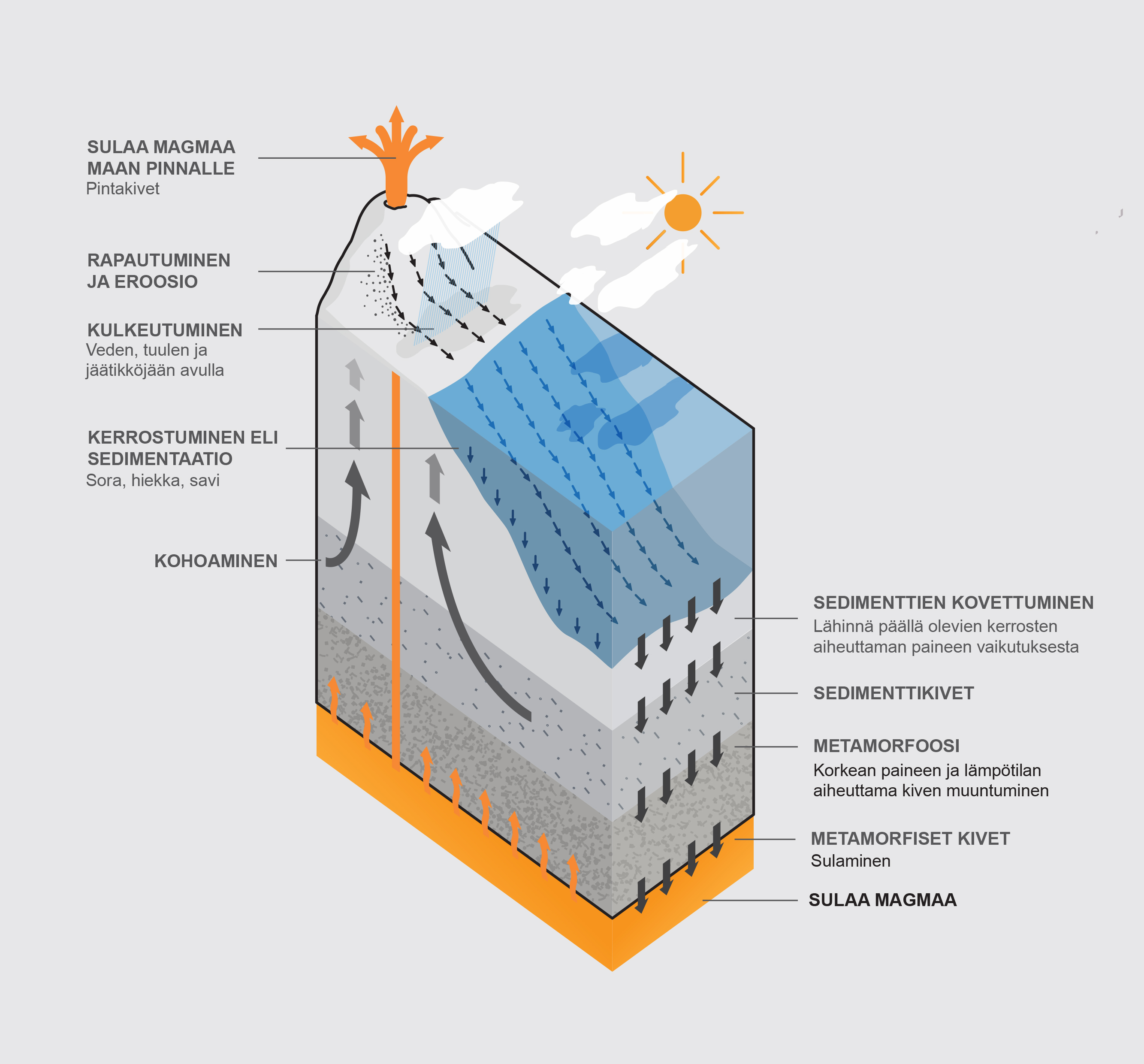
Figure 2. High circulation of a substance (translation will be included asap)
Primary clays are residually deposited clay minerals, such as kaolin and bentonite. Primary clays are often the purest clays. Secondary clays are clay minerals that have migrated to other areas of origin and contain varying amounts of impurities. Secondary clays include, for example, ball clays and natural clays.
Most naturally occurring minerals are a combination of silicon (Si) and oxygen (O), but they often contain other common crustal elements such as iron and aluminum. These substances are called silicate minerals. Silicates are the key raw materials for ceramics. By combining them and firing them at high temperatures, different clay bodies and glaze surfaces are obtained.
Theoretically, the chemical composition of a clay crystal is expressed by the formula: Al₂O₃ ⋅ 2SiO₂ ⋅ 2H₂O. Clay crystals are flat, hexagonal, microscopically small plates whose shapes and sizes vary according to different clay grades (Jylhä-Vuorio, 2003).
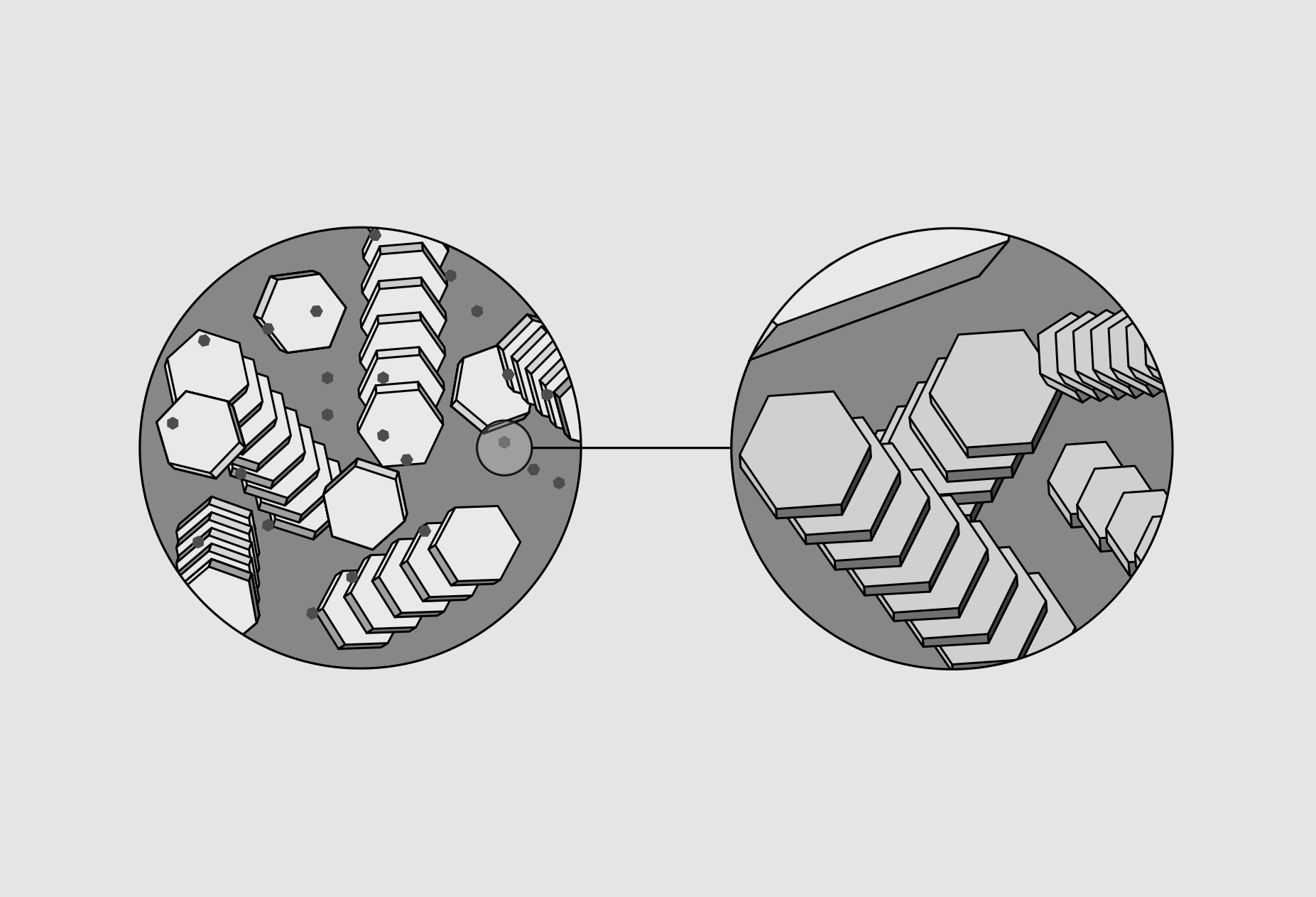
Figure 3. Clay crystal
Different clay bodies and plasticity
A mixture of clay minerals and other raw materials is called a clay body. Clay bodies can be found directly in nature (red clay, natural stoneware clays), but in practice most clay body mixtures are industrially produced. Typical properties of clay bodies are plastic and non-plastic.
Plasticity in physics is a property of matter in which the effect of force on its shape results in irreversible deformations. The plasticity of clay means that the clay body can withstand stretching and machining without tearing. The smaller the clay particles, the greater the plasticity they provide in the clay body.
A fine, plastic body is called fat (versus short). Because the fat and fine-particle body holds a lot of water, its drying shrinkage is high. When drying, a body with a large shrinkage rate causes defects in the object, such as cracks. To avoid defects, the clay bodies can be adjusted with non-plastic raw materials such as quartz, feldspar and crushed clay, i.e. chip or chamotte. Non-plastic raw materials reduce shrinkage during drying, i.e. drying shrinkage (Jylhä-Vuorio, 2003).
Plastic or non-plastic clay bodies are suitable for various manufacturing techniques. Plastic compounds are suitable for throwing, hand-building and molding. Compression powders and granules of non-plastic clay bodies are used in industrial production. Casting slip, or casting clays, are fluid mixtures, in which a form is usually made using a plaster mold.
The plasticity of different clay bodies is regulated by their raw material composition. Raw materials are divided into plastic and non-plastic raw materials. Plastic raw materials include, for example, kaolin, ball clays, red clays and bentonite. Non-plastic raw materials include feldspar, nepheline syenite, quartz, flint, grog and fibers.
Ceramics
Ceramics are clay fired at high temperatures. After drying, the clay can be converted back to plastic form by mixing it with water countless times, but if the clay is heated to a temperature above about 600 ° C, it becomes a hard substance that is no longer soluble in water and does not return to its plastic form. Because of this hardening, pottery is called an eternal material because it never decays, but at most shatters and crumbles.
Ceramic change means that clay never ever returns to a plastic body, its minerals have changed their chemical crystal structure under the influence of heat and clay has changed into a new, durable material, i.e. ceramics.
The term ceramic is used for inorganic materials made at high firing temperatures. In everyday language, the word ceramic often refers to the so-called technical ceramics, which are new materials developed in recent decades. Technical ceramics include very strong materials that are used to replace e.g. metals.
The term ceramics is used for objects made from clay-based raw materials that have undergone a ceramic firing process. Ceramics can be divided into two groups according to the degree of processing of the raw materials used: coarse ceramics and fine ceramics. Conventional coarse ceramic products include various bricks and brick elements. Fine ceramics can be considered to include, for example, household and sanitary ceramics.Glaze
Glaze is a thin layer of glass on the surface of the ceramic body. Glazes are named according to their composition, appearance, firing temperature and firing method. They can be glossy, matte, translucent or opal in visual properties. The glazes may have crystals or may be completely clear. Glazes can be stained with metal oxides and pigments. The raw materials they contain or clay base can affect the properties of the glaze. Firing conditions also affect the appearance of the glaze.
The glaze is selected according to the firing temperature of the clay body used. For example, low-fire bodies are glazed with low-fire glazes (900–1180 °C) and high-fire clay bodies are glazed with high-fire glazes (1200–1400 °C).
Glaze is used for many different reasons. In utensils, the glazed surface is more hygienic and easier to keep clean than the unglazed surface. In addition, the glaze improves the wear resistance of the object and makes the object waterproof. There may also be purely aesthetic reasons for using glaze. For example, it can improve the appearance of an object and smooth out defects visible in the body. In art ceramics, the visual properties of the glaze can go beyond usability requirements.
The properties of the glaze are substantially affected by the raw materials used, the clay body used, the firing method, the firing temperature, the thickness of the glaze layer and the composition of the glaze mixture. By their nature, glazes are not precise chemical compounds, but mixtures whose melting is affected by several simultaneous eutectic reactions. The properties of the glaze are regulated by changing their chemical composition, i.e. the amount of compounds in relation to each other. The basic raw material and glazing agent of the glaze is silica SiO₂ obtained from quartz sand. In addition to quartz, fluxes are added to the glaze to lower the melting temperature, usually in the form of feldspar minerals and frit. In addition to these, many other ceramic oxides act as glaze fluxes.
Glazes are divided into two groups according to the firing temperature: low-fire glazes 900–1180 °C and high-fire glazes 1200–1400 °C. The breakdown is indicative. Both groups include glossy, semi-matte, and matte glazes depending on the surface melt.
Ceramic clay-based coatings
A simple surface treatment for ceramics can be done by burnishing. Burnishing takes place when the object is in the raw or greenware stage and the surface is dry. The surface is rubbed, for example, with the bottom of a spoon or a smooth stone. In this way, the porous surface of the clay becomes shiny and dense as the smallest particles pack in a dense layer on the surface of the object. (Salmenhaara, 1983). Polishing with burnishing is only suitable for low temperatures, as at high temperatures the melting reactions of the clay body remove the resulting shiny effect.
A slip called Terra Sigillata is closer to an engobe than glaze, although it sometimes resembles a very thin layer of transparent glaze. Terra Sigillata is made by separating the finest part of the clay. (Salmenhaara, 1983). It was used to decorate high-quality pots in ancient Greece. The result was a dense, glossy, water- and dirt-repellent surface with very precise, detailed Illustrations.
Engobe is a generic name for clay slip, which often consists essentially of the same raw materials as the clay body of the object itself. An engobe has a wide range of applications and is suitable for both low and high fire. Engobes can, for example, be painted, sprayed or dipped for surface application of an object. It is colored by mixing metal oxides or pigments. (Salmenhaara, 1983). An engobe can be added to the surface of an object when it is leather-hard, completely bone-dry, or bisque fired. The range of possibilities of engobe surface decoration are very wide, and it is possible to layer different colored translucent glazes to create a wider range of colors and surfaces.
References
Brinck, J. (2019). Keramiikan valmistuksen prosessi sekä valmistus- ja koristelutekniikoita [Unpublished teaching material]. Aalto university.
Currie, I. (e.p.) Understanding Glazes. http://glazes.org/
Hortling, A. (e.p.) Keramiikan värit ja pigmentit. University of Art and Design Helsinki. Retrieved 11/27/2020, http://www.airihortling.fi/
Hortling, A. (e.p.) Lasite ja lasittaminen. University of Art and Design Helsinki. Retrieved 11/27/2020, http://www.airihortling.fi/
Jokinen, E. (2019). Keramiikan kemiaa, empiirinen kaava [Unpublished Teaching Material]. Aalto university.
Jokinen, E. (2019). Lasiteluento [Unpublished teaching material]. Aalto university.
Jokinen, E. (2019). Saviluento [Unpublished teaching material]. Aalto university.
Jylhä-Vuorio, H. (2003). Keramiikan Materiaalit. Kuopio: Taitemia.
Lautenbacher, N. (2016). Keramiikan materiaalit ja poltto [Unpublished teaching material]. Aalto university.
Pelkonen, T. (2009). Massojen ja lasitteiden valmistus [Unpublished teaching materials]. Aalto university.
Salmenhaara, K. (1983). Keramiikan Massat, Lasitukset, Työtavat. Keuruu: Otava.Reading recommendations
Au, D. (n.d.) Glazy database. https://glazy.org
Cordes, U. (2019). Online Glaze Calculator. https://www.online-glaze-calculator.com/Calculator/fr2.php
Britt, J. (2018). Ceramics Arts Network. https://ceramicartsnetwork.org/daily/ceramic-glaze-recipes/glaze-chemistry/triaxial-blend-test-pottery-glazes/
Daly, G. (2020). Ceramics Arts Network. https://ceramicartsnetwork.org/daily/ceramic-glaze-recipes/glaze-chemistry/line-blends-a-surefire-way-to-build-your-understanding-of-ceramic-glaze-materials/Images and illustrations
Figure 1. Van der Bent, M. (2013). Cour royale de Tiébélé (jpeg) Retrieved 2.12.2020, https://search.creativecommons.org/photos/d74bcf0e-a1cc-47d2-a058-dec1f1c98a09Figure 2. Kinnunen, A. (2020). Different Clay Materials. [Photo].
Figure 3. Kerimov, N. (2020). High circulation of a substance. [Illustration].
Figure 4. Kerimov, N. (2020). Clay Crystal. [Illustration].
